In the midst of the hectic laboratory work, validations may sometimes feel like a meaningless burden. It would be so nice to get them quickly out of the way and focus on the real work.
 But as we know, validations are far from being meaningless. From patient safety point of view, it is crucial that the tests used in a laboratory give relevant information about a patient condition, work consistently and reliably, and their performance characteristics are well understood.
But as we know, validations are far from being meaningless. From patient safety point of view, it is crucial that the tests used in a laboratory give relevant information about a patient condition, work consistently and reliably, and their performance characteristics are well understood.
Planning, conducting, reporting and reviewing validations according to regulatory and quality requirements demands serious commitments and resources. We trust that with Validation Manager you will do validations better and save your valuable time. Here’s a small recap on how we can help you to streamline your validation workflow.
Facilitating validation planning
To make sure that validations meet required criteria, organizations typically have a set of standard operation procedures (SOPs) to guide validation planning. Project templates enable adding these guidelines into Validation Manager. Company managers can define multiple templates for different purposes (e.g. light verification, monthly follow-up, in-house validation) to give standardized lists of studies to be conducted, all of which can be named by your familiar conventions. When a new validation project begins, users can then select a pre-made project template to easily create the required study set and get instructions for performing them.
Whether you use project templates or not, we have more ways to support you when you plan your validations.
Planning a validation requires a set of decisions. Validation Manager planning features offer a checklist about things to be considered to help you decide how to conduct your validations. In some studies we also help you in figuring out, how growing or reducing the sample amounts, replicates etc. affects the scope of your examination. And as soon as you start importing your data, the confidence intervals on the report will give you more information about the adequacy of your data set.
Supporting your work with validations
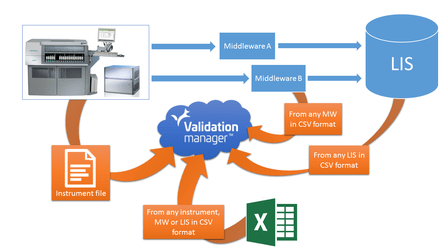
Finding, capturing and transferring measurement data is oftentimes a cumbersome and error-prone phase in validations. Something which you have to repeat from day to day and from project to project.
In Validation Manager, we have introduced versatile ways to capture data directly from the instruments and IT systems without a need to pre-process, filter or otherwise prepare data in Excel. You can simply export the desired data from laboratory instrument, middleware or LIS systems and import it directly to Validation Manager minimizing the potential data handling errors.
Recently Validation Manager Data import has been entirely redesigned. It is faster, gives more flexibility and copes with significantly larger amount of data. Data can be imported also for multiple instruments at the same time through LIS file import.
One of the core building blocks of Validation Manager is the fully automated result calculation engine. This means that all validation results with relevant statistics are automatically calculated based on the study plan and captured data. The clever statistical tools behind provide information about the reliability of your achieved results, warn about discrepancies in the data and indicate potential outliers and other statistical anomalies. You may also exclude measurements or mark data points as outliers, and it is easy to track what has been excluded and why.
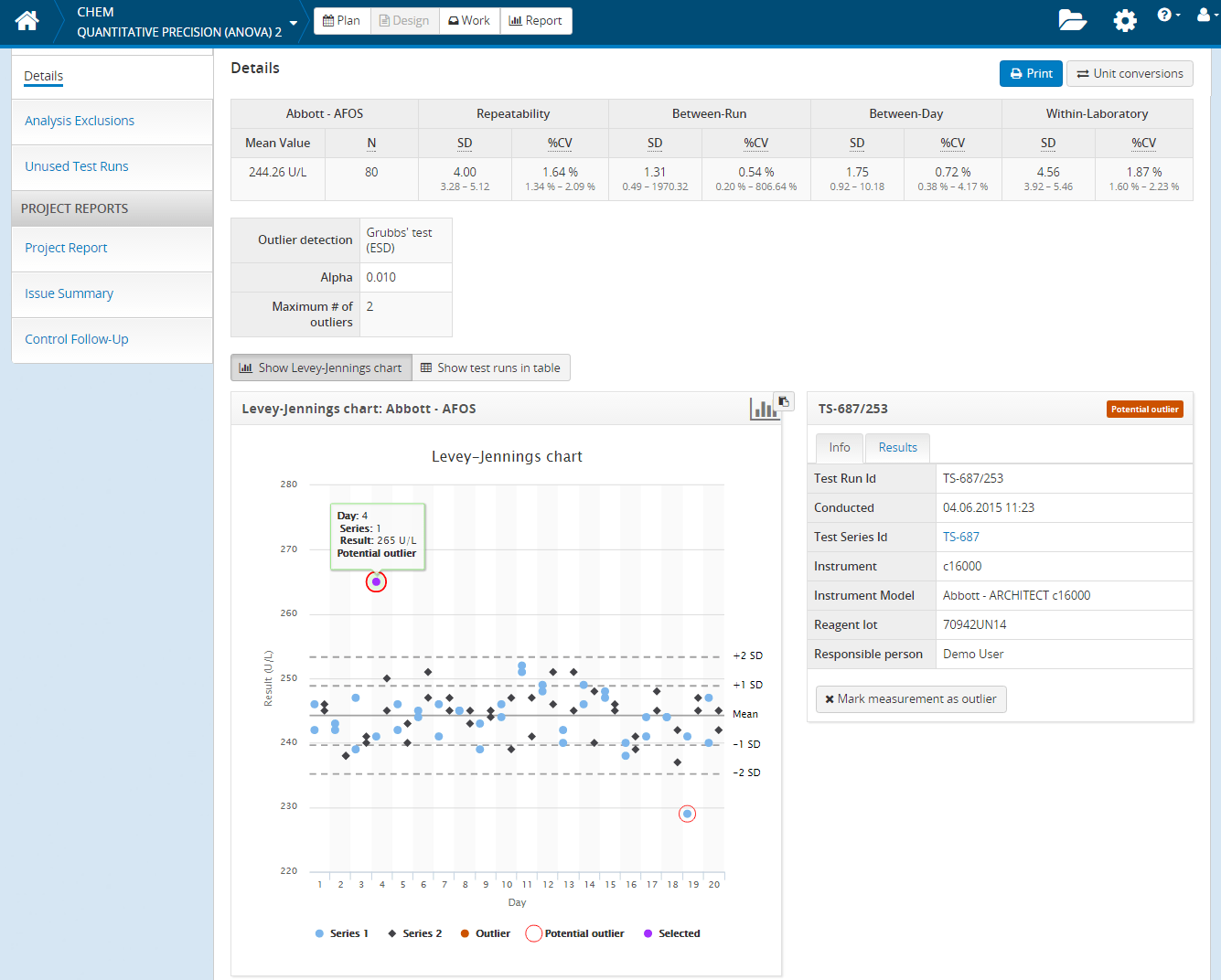
Thanks to fluent data capture and automated result calculation, you can easily track the progress of your work even on daily basis and view how your results build up. All this will save your valuable time and budget while working with validations. It gives you control to spot problems early and act on them before all your validation measurements have been conducted. And in best cases, if your samples have less variability than expected, you may be able to stop data collection earlier than you expected.
Easy reporting of validations
Validation Manager has far more holistic and sophisticated approach to validations compared to tools relying on Excel analysis. While you might be asked to provide somewhat more information about your samples and other variables while setting up the system, it pays off when you start examining the validation results.
If your result data raises questions, you don’t need to go through a bunch of Excel sheets and notebook pages to find sample and measurement information. Just click a link on your Report page and you can easily access specific information behind the results. Study reports give you comprehensive tools for filtering results e.g. based on analyte, sample type and other provided classification factors.
When validation work is done and results have been processed, you probably need to compile a printable project report. This is where Validation Manager really comes into its own. Instead of manually creating or copying graphs and tables one by one, you can simply download all relevant graphs and tables in one Word document that is built on your organization’s document template. After you have added your specific conclusions by yourself, everything is done. Whenever you need to get back to your data, you can easily find it in Validation Manager where all the data is stored and maintained centrally.
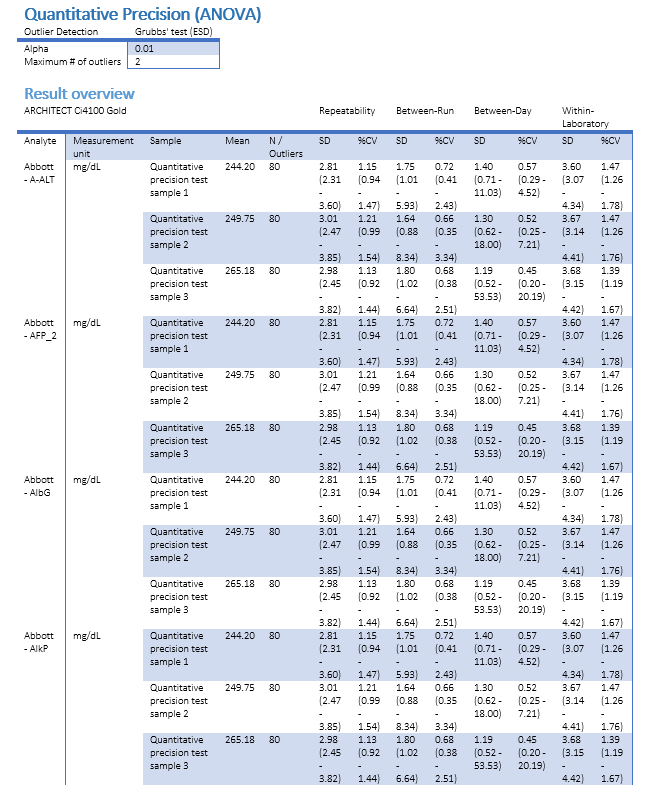
Lately we’ve been adding some nice enhancements to reporting features
- Precision study (ANOVA) overview report will summarize results for multiple analytes on one table
- Precision study (ANOVA) analysis speed is 2x faster than before
- Page number estimate for project report indicates the size of the expected end-report
- Analytical sensitivity (Probit) study has been expanded to show average Ct values for each reagent concentration
Accepting validations
How do you manage which validation projects are done, which are still pending some data or waiting for final acceptance? How do you find and gather validation related information for your auditing or accreditation needs?
If your organization has a review process for validations, you may want to utilize Validation Manager workflow for managing review process. You can also archive ready projects to simplify working with unfinished projects and easily find the finished validation reports.
Having all the relevant validation data available digitally in one place and organized properly is likely to ease your life. Whenever you have to come back to any past validation data, continue your previously conducted validation actives, work with scientific publications or prepare for forthcoming audits and accreditations, you can find it all in Validation Manager.
This was just a short review of features and benefits to help you towards smoother validations. There’s so much more – and even more coming. As you may already know, our development philosophy is to work very closely with our customers and end-users. By providing us feedback, you will help us to help you. We´re looking forward to hearing from you!
Accomplish more with less effort
See how Finbiosoft software services can transform the way your laboratory works.