
One of the benefits of using Validation Manager for your verifications is that setting performance goals saves you a lot of effort in manually examining all your reports. You can import all your verification results directly from your LIS and then immediately see on the report whether some analytes didn’t reach your goals.
Selecting goals for your verifications is a crucial step in standardized verifications, and so far, it has been a bit laborious to set them separately for each study. In some cases, you’ve been able to save this trouble by copying the plan from a previous verification, and you can of course always ask someone to review your study goals, but still, the workflow for making sure that the goals are correct has not been as smooth as it could.
Especially in bigger organizations, the goal management should be centralized, so that quality managers could set the goals, and then people doing verifications could easily use them. And even in small laboratories, if the goals are based on EFLM or some other biological variation database, it would make sense to define these once and then just use them in all studies.
Solution — the new Quality Management module
To solve this issue, we have started to develop a new module for quality managers. In the first phase, this module will enable centralized goal management. Quality managers can save goal sets for different purposes, and then people planning their verifications can simply select the goal set they need.
Later there will be more exciting features to help quality managers in their work, for example, an overview to the performance of all their laboratories, instruments, and analytes. Once the main features of the new module are ready, it will be offered as an optional module for Validation Manager. This way, organizations who do not consider it worth the price to get better visibility to their laboratory quality can still get the benefits of all basic validation and verification features of Validation Manager.
Try it out today and tell us how you like it
During the development of the main features of this module, we offer a trial period for all our existing customers. To make it easier to get started, here’s a short introduction to the new features. Check out the user guide for a more detailed description.
In Validation Manager Settings, quality managers can add new goal sets for different purposes. You can gather all the EFLM goals into one goal set, or you can divide it into multiple sets to manage different analyte groups or locations separately, enabling each department to manage their own goals. You can also have different goal sets for different types of studies. For example, you can have one goal set for your periodic parallel instrument comparisons.
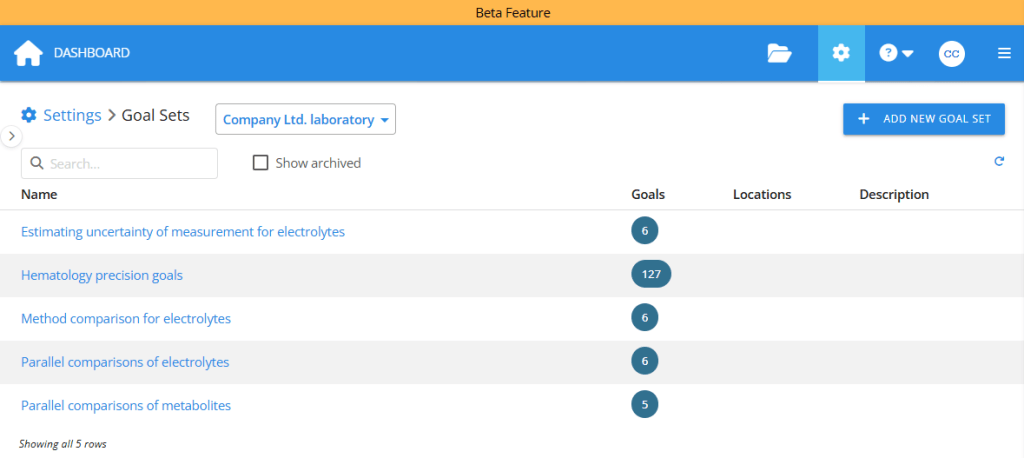 When users plan their studies, they can import goals simply by pressing a button and selecting the correct goal set. For now, this feature is only available for quantitative studies Comparison, Precision (ANOVA), and Quantitative Accuracy.
When users plan their studies, they can import goals simply by pressing a button and selecting the correct goal set. For now, this feature is only available for quantitative studies Comparison, Precision (ANOVA), and Quantitative Accuracy.
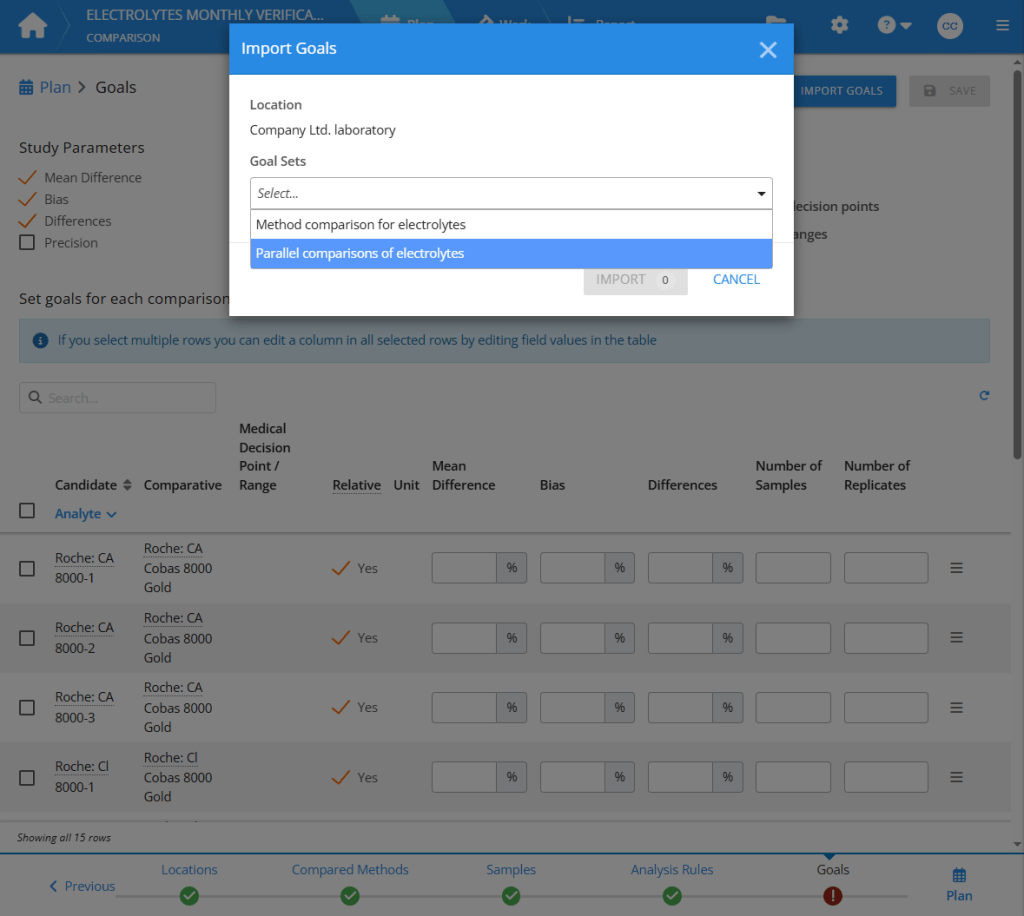 Try the new features and see how much they help you in planning your verifications! If anything seems to be missing or would work better some other way, we appreciate all feedback for further development of the quality management module.
Try the new features and see how much they help you in planning your verifications! If anything seems to be missing or would work better some other way, we appreciate all feedback for further development of the quality management module.
Accomplish more with less effort
See how Finbiosoft software services can transform the way your laboratory works.





